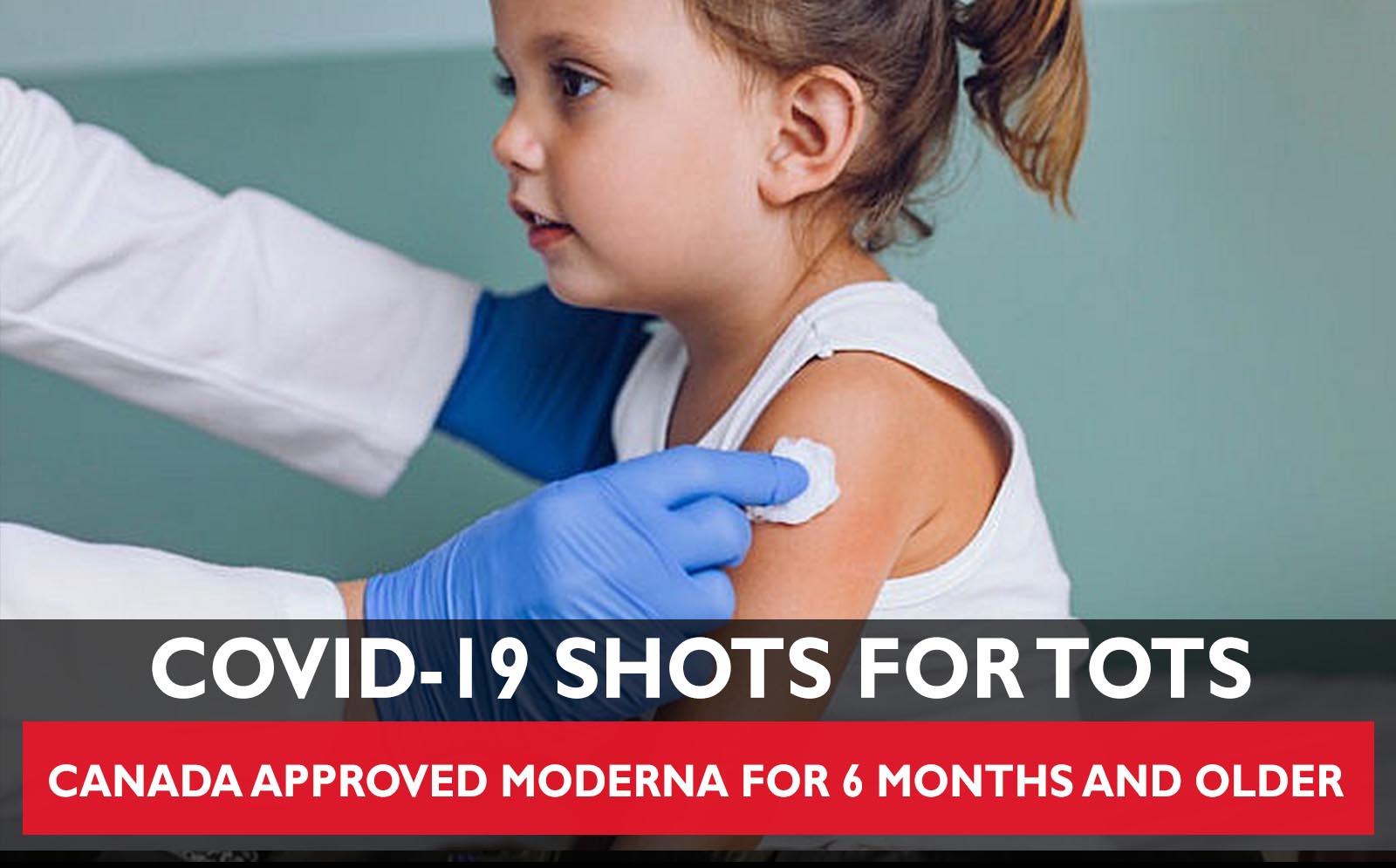
Health Canada says it has approved the use of Moderna’s COVID-19 vaccine for children as young as six months old.
In a July 14 statement, the federal health regulator announced it has authorized the use of Moderna’s Covid-19 vaccine for children 6 months to 5 years of age, making Moderna the first pharmaceutical company permitted to provide their vaccine to Canada’s youngest kids. The vaccine requires two doses given about four weeks apart.
“Health Canada has authorized a two-dose primary series half the dose authorized for children 6 to 11 years of age, and one-quarter of the dose authorized for people over 12 years of age,” said the statement.
The approval also means that nearly two million children in Canada could be eligible for the shots, depending on the availability of the vaccine determined by each province.
Health Canada previously said it received a submission from Pfizer-BioNTech on June 23, requesting authorization of its vaccine for children between six months and four years old. The submission is still under review.
“After a thorough and independent scientific review of the evidence, the department has determined that the vaccine is safe and effective at preventing COVID-19 in children between 6 months and 5 years of age,” the department said.
Ontario Chief Medical Officer Kieran Moore said not everyone should get COVID-19 vaccine due to risk of myocarditis. He said there is a "1 in 5000" chance of getting myocarditis, adding "you'd have to have that discussion on the risk-benefit, for a young, healthy person." pic.twitter.com/Wnkq5wMEPZ
— Canadian Riley (@TiaRileyCanada) July 14, 2022
The vaccine is approved only days after Ontario’s Chief Medical Officer Dr. Kieran Moore announced that “We know there is a risk, a very small risk, 1 in 5,000 that may get myocarditis, for example, and you’d have to have that discussion on the risk-benefit of complications from the vaccine versus the benefit of decreased hospitalization for a young healthy person.”